Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O in Fe
O in Fe O
O film on Fe formed in an NaOH solution containing oxidants
film on Fe formed in an NaOH solution containing oxidantsHaruna, Takumi*; Miyataki, Yuki*; Hirohata, Yohei*; Shibata, Toshio*; Taniguchi, Naoki; Tachikawa, Hirokazu*
Zairyo To Kankyo, 67(9), p.375 - 380, 2018/09
This research aimed to confirm the formation of Fe O
O film on Fe immersed in aqueous 45 mass% NaOH solution containing some oxidants at the boiling temperature, to recognize the optimum immersion time for the formation of thick and protective film, and to reveal the absorption behavior of D
film on Fe immersed in aqueous 45 mass% NaOH solution containing some oxidants at the boiling temperature, to recognize the optimum immersion time for the formation of thick and protective film, and to reveal the absorption behavior of D O in the Fe
O in the Fe O
O film at room temperature. The results were obtained as follows. It was confirmed that Fe
film at room temperature. The results were obtained as follows. It was confirmed that Fe O
O film was formed on Fe immersed in the NaOH solution for a time more than 0.6 ks, and the film thickness increased parabolically with an increase in the immersion time. D
film was formed on Fe immersed in the NaOH solution for a time more than 0.6 ks, and the film thickness increased parabolically with an increase in the immersion time. D O absorption test was carried out to the films formed in the NaOH solution for immersion times of 1.2 and 3.6 ks. An amount of D
O absorption test was carried out to the films formed in the NaOH solution for immersion times of 1.2 and 3.6 ks. An amount of D O absorbed into the film increased with an increase in an absorption time up to 1000 ks, and an absorption time more than 1000 ks made an amount of D
O absorbed into the film increased with an increase in an absorption time up to 1000 ks, and an absorption time more than 1000 ks made an amount of D O constant. The constant amount of D
O constant. The constant amount of D O was larger for the film formed on Fe immersed in the NaOH solution for 3.6 ks than that for 1.2 ks. The transient of the amount of D
O was larger for the film formed on Fe immersed in the NaOH solution for 3.6 ks than that for 1.2 ks. The transient of the amount of D O absorbed into the film was analyzed on the basis of Fick's law for diffusion, and diffusion coefficients of D
O absorbed into the film was analyzed on the basis of Fick's law for diffusion, and diffusion coefficients of D O were obtained to be 5.1
O were obtained to be 5.1 10
10 cm
cm s
s and 9.9
and 9.9 10
10 cm
cm s
s for the films formed for 1.2 and 3.6 ks, respectively. Therefore it was estimated that the diffusion coefficient of the Fe
for the films formed for 1.2 and 3.6 ks, respectively. Therefore it was estimated that the diffusion coefficient of the Fe O
O film was in the region from 5.1
film was in the region from 5.1 10
10 cm
cm s
s to 9.9
to 9.9 10
10 cm
cm s
s .
.
Iriya, Keishiro*; *; Fujita, Hideki*; Kubo, Hiroshi*
JNC TJ8400 2000-034, 212 Pages, 2000/02
Cementious materials and highly compacted bentnite are expectable candidates as materials of TRU waste repositories. It was pointed out that Bentonite might be changed to Zeolite and surrounding rock might be altered by high alkalinity water flow, since cement hydrate leached to pore water of cement and it was changed to alkaline. Transportation of radio-nuclides might be accelerated by organic materials, such as super plasticizer, and nitlate which is contained in nuclear wastes. It was concluded by previous studies that rock and bentonite is stable in alkaline water which pH is less than 10.5. The new type of low alkalinity cement with high silica fume and fly ash content which could keep pH below 11.0 was developed and its performance has been assessed. However since Zeolitation and ilitation were reported upon deterioration of bentonite bated in certain condition, it should be assessed by long term experiment. Since Capacity of keeping integrity of bentonite hasn't been directly checked by experiments upon the developed new type of low alkalinity cement it should be done. Although amount of leaching organic was quantitatively and experimentally assessed at an early age, effect of changing of amount and shape hasn't assessed in leaching of radio nuclides. Although it is pointed out that deterioration of cementitious materials isn't accelerated by condensed nitrate solution at early period after closure, it is considered that it might be accelerated corresponding to chemical composition in case of decrement of concentration of nitrate. In this study, deterioration of materials will be assessed in detail in order to feed back the results to assessment of transportation of radio nuclides. Long term deterioration of bentonite by leaching water of cement will be experimentally assessed, and deteriorating test of bentonite will be carried out by leaching water of low alkalinity cement. Amount of organic and component of it will be measured. Furthermore ...
Iriya, Keishiro*; *; Kubo, Hiroshi*; Fujita, Hideki*
JNC TJ8400 2000-033, 95 Pages, 2000/02
Cementious materials and highly compacted bentnite are expectable candidates as materials of TRU waste repositories. It was pointed out that Bentonite might be changed to Zeolite and surrounding rock might be altered by high alkalinity water flow, since cement hydrate leached to pore water of cement and it was changed to alkaline. Transportation of radio-nuclides might be accelerated by organic materials, such as super plasticizer, and nitrate which is contained in nuclear wastes. It was concluded by previous studies that rock and bentonite is stable in alkaline water which pH is less than 10.5. The new type of low alkalinity cement with high silica fume and fly ash content which could keep pH below 11.0 was developed and its performance has been assessed. However since Zeolitation and ilitation were reported upon deterioration of bentonite bated in certain condition, it should be assessed by long term experiment. Since Capacity of keeping integrity of bentonite hasn't been directly checked by experiments upon the developed new type of low alkalinity cement it should be done. Although amount of leaching organic was quantitatively and experimentally assessed at an early age, effect of changing of amount and shape hasn't assessed in leaching of radio nuclides. Although it is pointed out that deterioration of cementitious materials isn't accelerated by condensed nitrate solution at early period after closure, it is considered that it might be accelerated corresponding to chemical composition in case of decrement of concentration of nitrate. In this study, deterioration of materials will be assessed in detail in order to feed back the results to assessment of transportation of radio nuclides. Long term deterioration of bentonite by leaching water of cement will be experimentally assessed, and deteriorating test of bentonite will be carried out by leaching water of low alkalinity cement. Amount of organic and component of it will be measured. Furthermore ...
Akashi, Masatsune*; Fukaya, Yuichi*; Asano, Hidekazu*
JNC TJ8400 2000-015, 46 Pages, 2000/02
Difference of hydrogen generation phenomena on the surface of the Steels were not observed between carbon steel, atmospheric corrosion resisting steel and 5%-Ni steel. Rust layer was formed on these three-type of steels by steam oxidation method. And the chemical composition of the rust for the steels were basically two (2) layers structure for the previous two steels as hematite (Fe O
O ) based for the outer layer and magnetite (Fe
) based for the outer layer and magnetite (Fe O
O ) based for the inner layer. And for the last steel, it had three (3) layer in the rust as hematite (Fe
) based for the inner layer. And for the last steel, it had three (3) layer in the rust as hematite (Fe O
O ) based for the outer layer, magnetite (Fe
) based for the outer layer, magnetite (Fe O
O ) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
Akashi, Masatsune*; Fukaya, Yuichi*; Asano, Hidekazu*
JNC TJ8400 2000-014, 22 Pages, 2000/02
Difference of hydrogen generation phenomena on the surface of the Steels were not observed between carbon steel, atmospheric corrosion resisting steel and 5%-Ni steel. Rust layer was formed on these three-type of steels by steam oxidation method. And the chemical composition of the rust for the steels were basically two(2) layers structure for the previous two steels as hematite(Fe O
O ) based for the outer layer and magnetite(Fe
) based for the outer layer and magnetite(Fe O
O ) based for the inner layer. And for the last steel, it had three(3) layer in the rust as hematite(Fe
) based for the inner layer. And for the last steel, it had three(3) layer in the rust as hematite(Fe O
O ) based for the outer layer, magnetite(Fe
) based for the outer layer, magnetite(Fe O
O ) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
Sumiyama, Morio*
JNC TJ8400 2000-009, 138 Pages, 2000/02
To evaluate corrosion behavior of carbon steel, a candidate materials of overpack, buried in soil for a long time, the water pipes buried in freshwater clay for a long time we digged out and the soil environment and the corrosion weight loss of pipes have been researched. From the results, a corrosion model (an empirical equation), an oxygen reduction reaction rate-determing step type, of carbon steel buried in soil was introduced. The corrosion data of under ground pipe collected by the Japan Community Gas Associations was used to increase reliability of the corrosion model equation. These data are one of researches of corrosion behavior of carbon steel buried in soil for a long time studied by at home and abroad. 38 samples buried freshwater clay were selected in 171 samples. With estimating the corrosion velocities and the soil environment factors of the above data, the maximum depth of pit corrosion was calculated by the statistical method of the extreme values using the area of overpack as the recurrent time. The correlation between the soil environment factors and the corrosion weight loss was obtained by the correlation analysis. The corrosion model of the maximum depth of pit corrosion at 0.99 of cumulative probability was compared between the under ground pipe data and the above data. On the reference data and the above data, the corrosion model equation; H = aY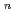 was compared with the maximum depth of pit corrosion at 0.99 cumulative probability. The data of water pipes and community gas pipes at 0.99 cumulative probability showed the reasonable values when these data were compared with the reference data. So that the model was proved as a good corrosion model m the neutral low dissolved oxygen environment.
was compared with the maximum depth of pit corrosion at 0.99 cumulative probability. The data of water pipes and community gas pipes at 0.99 cumulative probability showed the reasonable values when these data were compared with the reference data. So that the model was proved as a good corrosion model m the neutral low dissolved oxygen environment.
Imai, Jun*
JNC TJ8400 2000-008, 196 Pages, 2000/02
The objective of this research is to make clear long-term alteration processes of bentonite contacting with concrete under a repository condition for radioactive waste. The Uzu tunnel in yamagata prefecture in Japan, constructed during the term of December of 1963 to July 1967, was selected as an appropriate natural analogue: the tunnel wall was made of portland cement and which has been contacting with a bentonite bed during  32 years. Sample analyses indicated that the original bentonite was Na
32 years. Sample analyses indicated that the original bentonite was Na -type and it changed to Ca
-type and it changed to Ca -type in the range of a few millimeters from the contact. Although a Ca
-type in the range of a few millimeters from the contact. Although a Ca leaching was also observed from the concrete near the contact, neither transformation to zeolite nor to illite was recognized. On the other hand, sulfur increased and ettringite (3CaO
leaching was also observed from the concrete near the contact, neither transformation to zeolite nor to illite was recognized. On the other hand, sulfur increased and ettringite (3CaO  Al
Al O
O
 3CaSO
3CaSO 4
4  32H
32H O) was recognized in the concrete within the depth about 30 mm from the contact.
O) was recognized in the concrete within the depth about 30 mm from the contact.
Ishikawa, Hiroki*; Tada, Eiji*; Nishikata, Atsushi*; Taniguchi, Naoki; Tachikawa, Hirokazu*
no journal, ,
In current concept of geological disposal of high-level radioactive waste, the vittrified wastes are stored in metallic container which is called overpack, and then disposed in deep underground. Similarly in direct disposal of spent nuclear fuel, the fuel assemblies are disposed in deep underground contained in metallic disposal container. In order to ensure the long term integrity and safety of overpack or disposal container, corrosion monitoring was performed using electrochemical impedance spectroscopy (EIS) technique.
Nishioka, Tsuyoshi*; Ogawa, Soma*; Tsuchiya, Hiroaki*; Fujimoto, Shinji*; Taniguchi, Naoki; Dobashi, Ryuta*
no journal, ,
no abstracts in English
Haruna, Takumi*; Miyataki, Yuki*; Hirohata, Yohei*; Taniguchi, Naoki; Tachikawa, Hirokazu*
no journal, ,
no abstracts in English
Nishioka, Tsuyoshi*; Tsuchiya, Hiroaki*; Fujimoto, Shinji*; Tachikawa, Hirokazu*; Taniguchi, Naoki
no journal, ,
no abstracts in English
 irradiation
irradiationYukawa, Takuji*; Inoue, Hiroyuki*; Kojima, Takao*; Tachikawa, Hirokazu*; Taniguchi, Naoki
no journal, ,
no abstracts in English
Inoue, Hiroyuki*; Yukawa, Takuji*; Kojima, Takao*; Tachikawa, Hirokazu*; Taniguchi, Naoki
no journal, ,
no abstracts in English
Kai, Itsuki*; Nakanishi, Yuki*; Taniguchi, Naoki; Dobashi, Ryuta*; Hirohata, Yohei*; Haruna, Takumi*
no journal, ,
no abstracts in English
Watari, Shingo*; Kitayama, Ayami; Mitsui, Seiichiro; Taniguchi, Naoki; Kimura, Wataru*; Kajiyama, Hiroshi*
no journal, ,
The application of pure copper is being considered for the purpose of extending the life-time of disposal canisters in the direct disposal of spent fuel. Pure copper generally shows little corrosion development in aqueous solution environments with low oxygen concentrations due to its thermodynamic stability, but pure copper loses thermodynamic stability and corrosion develops depending on the sulfides conditions. In this study, immersion tests and U-bend tests were conducted to understand the corrosion progress behavior and stress corrosion cracking behavior of pure copper under the condition of sparging gas with various hydrogen sulfide concentrations to simulate the disposal environment where sulfide exists.
Miyoshi, Yuma*; Inoue, Hiroyuki*; Mitsui, Seiichiro; Dobashi, Ryuta*
no journal, ,
In Japan, research and studies on direct disposal of spent fuel as an alternative disposal option are underway in order to secure a wide range of options. For direct disposal, a copper-carbon steel composite vessel covered with pure copper, which is expected to provide longer confinement, has been considered in consideration of the time required for sufficient attenuation of C-14, one of the dominant radionuclides in the safety assessment. In this study, the effect of HCO
 on the cathodic reaction of pure copper in a fresh groundwater environment with a low oxygen atmosphere was investigated. From the results of polarization measurements using pure copper as a test electrode, it was inferred that HCO
on the cathodic reaction of pure copper in a fresh groundwater environment with a low oxygen atmosphere was investigated. From the results of polarization measurements using pure copper as a test electrode, it was inferred that HCO
 promotes the cathodic reaction, which is a hydrogen evolution reaction using H
promotes the cathodic reaction, which is a hydrogen evolution reaction using H as an oxidant, by buffering the pH change at the electrode interface in the relatively low current density range, which is assumed to be the cathodic current of corrosion. It is unlikely that HCO
as an oxidant, by buffering the pH change at the electrode interface in the relatively low current density range, which is assumed to be the cathodic current of corrosion. It is unlikely that HCO
 acts directly as an oxidant in the cathodic reaction of corrosion. The hydrogen evolution reaction rate on the pure copper surface is relatively low in comparison with the polarization measurements on pure iron and platinum.
acts directly as an oxidant in the cathodic reaction of corrosion. The hydrogen evolution reaction rate on the pure copper surface is relatively low in comparison with the polarization measurements on pure iron and platinum.